- A
- A
- A
ABUS
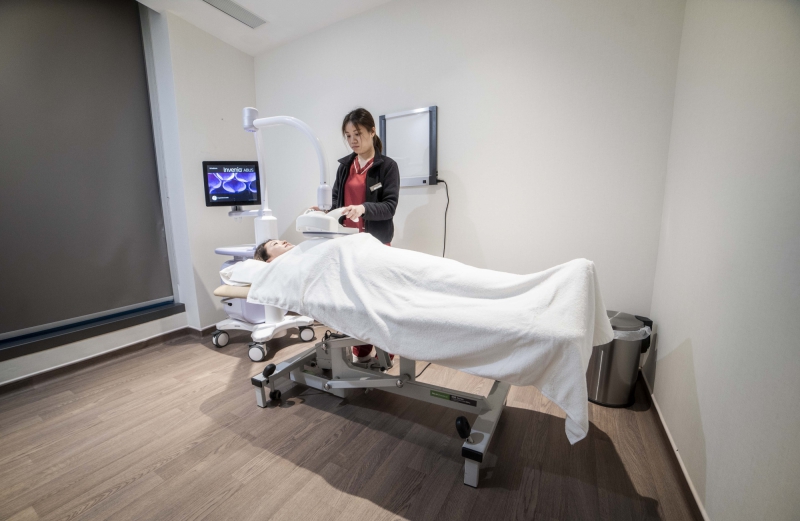
GE Invenia™ ABUS
GE Invenia™ ABUS is the only breast cancer screening technology approved by the FDA for detection in women with dense breast tissue.
GE InveniaTM ABUS breast cancer screening is specially developed to help doctors find cancers hidden in dense breast tissue, which may be missed by mammography.
The Reverse-Curve™ transducer enhances both patient comfort and breast coverage during the exam. The 15 cm ultra-broadband, wide field-of-view transducer automatically creates uniform compression across the entire breast for consistent, reproducible image quality independent of the operator. The convergent scan line geometry minimizes beam refraction. The result is deep penetration, sharp focus, resolution at depth and enhanced anatomical detail of complex breast tissue and structures.
GE Invenia™ ABUS is the only breast cancer screening technology approved by the FDA for detection in women with dense breast tissue.
GE InveniaTM ABUS breast cancer screening is specially developed to help doctors find cancers hidden in dense breast tissue, which may be missed by mammography.
The Reverse-Curve™ transducer enhances both patient comfort and breast coverage during the exam. The 15 cm ultra-broadband, wide field-of-view transducer automatically creates uniform compression across the entire breast for consistent, reproducible image quality independent of the operator. The convergent scan line geometry minimizes beam refraction. The result is deep penetration, sharp focus, resolution at depth and enhanced anatomical detail of complex breast tissue and structures.
